What are triacylglycerols, and how do they serve us?
This is an excerpt from Exercise Biochemistry 2nd Edition by Vassilis Mougios.
Triacylglycerols, or triglycerides, are the most abundant lipid category. They are the main components of animal (including human) fat, most of which is concentrated in adipose tissue; they are also the main components of vegetable oils. Because of this abundance, they constitute 90% to 95% of dietary fat. In both animals and plants, triacylglycerols serve mainly as energy depots.
A triacylglycerol consists of a glycerol unit and three fatty acid units. Glycerol (figure 5.12), also known as glycerin or glycerine, is a compound of three carbons and three hydroxyl groups, each of which connects with the carboxyl group of a fatty acid to form an ester linkage. Thus, every triacylglycerol (figure 5.12) contains three ester linkages, which makes it a triester.
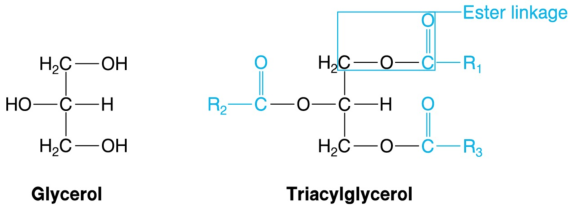
Figure 5.12 Glycerol and triacylglycerol. Triacylglycerols, the largest energy depot in living organisms, are triesters of glycerol and fatty acids. R1, R2, and R3 represent the aliphatic chains of the fatty acids, which usually differ. Acyl groups are shown in color.
Because there is a great variety of available fatty acids, and because any of them can be linked to any of the hydroxyl groups of glycerol, we end up with an even greater variety of triacylglycerols. Chances are that there will be both saturated and unsaturated fatty acids present in a single triacylglycerol. Therefore, it is not accurate to state that a certain food contains only saturated or unsaturated fat. Instead, one should state that a certain food contains primarily saturated or unsaturated fatty acids.
Triacylglycerols are hydrophobic, which is evident by the immiscibility of fats or oils with water. Moreover, triacylglycerols have low thermal conductivity, rendering the subcutaneous fat of animals an efficient insulator of their internal organs against cold exposure.
The part of a fatty acid connected to an oxygen of glycerol in a triacylglycerol is called an acyl group. This is where the term tri-acyl-glycerol comes from; hence it is more accurate than triglyceride. The acyl groups derive from the ion of a fatty acid by removal of O-, and they bear the name of the fatty acid with the ending -oyl in place of -ate. Thus, the acyl group of palmitate is called the palmitoyl group.
The difference in melting point between saturated and unsaturated fatty acids described in the previous section is reflected in triacylglycerols: The more saturated acyl groups they contain, the higher their melting point is. Triacylglycerols of animal origin have a high content of saturated acyl groups, which is why animal fat is solid at room temperature. Conversely, plant triacylglycerols have a high content of unsaturated acyl groups, which is why vegetable oils are liquid in the same conditions.
SHOP

Get the latest insights with regular newsletters, plus periodic product information and special insider offers.
JOIN NOW
Latest Posts
- How do I integrate nutrition education into PE?
- How does the support of friends and family influence physical activity?
- What makes the Physical Best approach unique?
- Strength training gimmicks . . . or not?
- How do vitamins and minerals support our bodies?
- Why do many people have difficulty losing weight?


